Abstract
Background Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired hematologic condition associated with significant morbidity, mortality, and impact on health-related quality of life (HRQoL). PNH leads to a wide range of symptoms, and patients feel the impact of their illness across physical, emotional, and social aspects of their lives. This study sought to document the impact of different treatments on helping alleviate the burden of disease, as reported by patients in the real-world setting.
Methods This mixed-methods study enrolled consenting, adult patients (> 21 years old) with PNH in the US for telephone depth interviews. Eligible participants were required to be currently treated with and have at least 3 months of treatment experience with pegcetacoplan.
Results Results from 15 patients (8 female and 7 male) are reported here. All patients had received treatment with a C5-inhibitor (C5-i) prior to switching to pegcetacoplan. Interviewed patients described living with PNH as frustrating, exhausting, and uncertain, leading to a significantly impacted overall HRQoL. Fatigue was frequently described as the most prominent symptom of PNH, causing patients to consistently feel the need to save their energy throughout days and weeks for essential and basic tasks. Of the N=15 patients who participated in the study, n=13 (87%) reported fatigue as their Most Bothersome Symptom due to PNH, as it frequently limits their ability to carry out normal daily activities and negatively impacts their HRQoL.
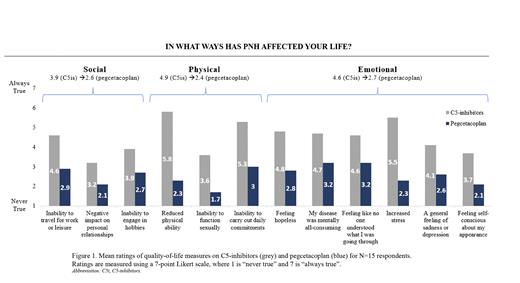
On average, patients reported a moderate quality of life overall while being treated with C5-is, with an average score of 4.4 on a 7-point Likert scale, where 1 is "very poor" and 7 is "excellent." Furthermore, patients reported not achieving their physical, emotional, and social HRQoL treatment goals while on C5-is. In particular, the metrics that resonated most with patients, as it relates to their experience while on C5-is, were continued reduced physical ability (i.e., ability to exercise, walk, or jog) (5.8 mean on a 7-point Likert scale, where 1 is "never true" and 7 is "always true"), increased stress (5.5), inability to carry out daily commitments (i.e., going to work or school) (5.3), and an overall feeling of hopelessness (4.8) (Figure 1).
All patients reported the impact of PNH on HRQoL after switching to pegcetacoplan. The mean improvement across these patients was 27% compared to C5-is (5.6 compared to 4.4 mean on C5-is on a 7-point Likert scale).
This improvement was also reported across overarching categories of physical (51% relative improvement, N=15), emotional (41% relative improvement, N=15), and social (33% relative improvement, N=15) HRQoL measures (Figure 1). Most notably, compared to ratings while on C5-is, patients experienced a 58% improvement in physical ability, 58% improvement in stress, 42% improvement in ability to carry out daily commitments, 41% improvement in feelings of hopelessness, and 52% improvement in ability to function sexually, on average, while on pegcetacoplan (Figure 1).
Conclusions Patients interviewed in this study continued to feel a negative impact of PNH on their HRQoL while receiving treatment with C5-is. After switching to pegcetacoplan, patients reported improvement across physical, emotional, and social measures of HRQoL, in addition to an increase in overall QoL. Capturing the Voice of the Patient is crucial to characterize the real-world patient experience and also to optimize clinical approaches to disease treatment and management.
Disclosures
Desai:Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Dingli:Alexion Pharmaceuticals: Consultancy; Apellis Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy; GlaxoSmithKline: Consultancy; Janssen Pharmaceuticals: Consultancy; Novartis: Consultancy; Takeda Pharmaceuticals: Consultancy; Sanofi S.A.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal